Effect of Gene Knockout of CEP290 using CRISPR-Cas 9 Technology on Xenopus tropicalis
DOI:
https://doi.org/10.59973/emjsr.67Keywords:
CRISPR CaS 9, Xenopus tropicalis, Gene knockout, Kidney defectsAbstract
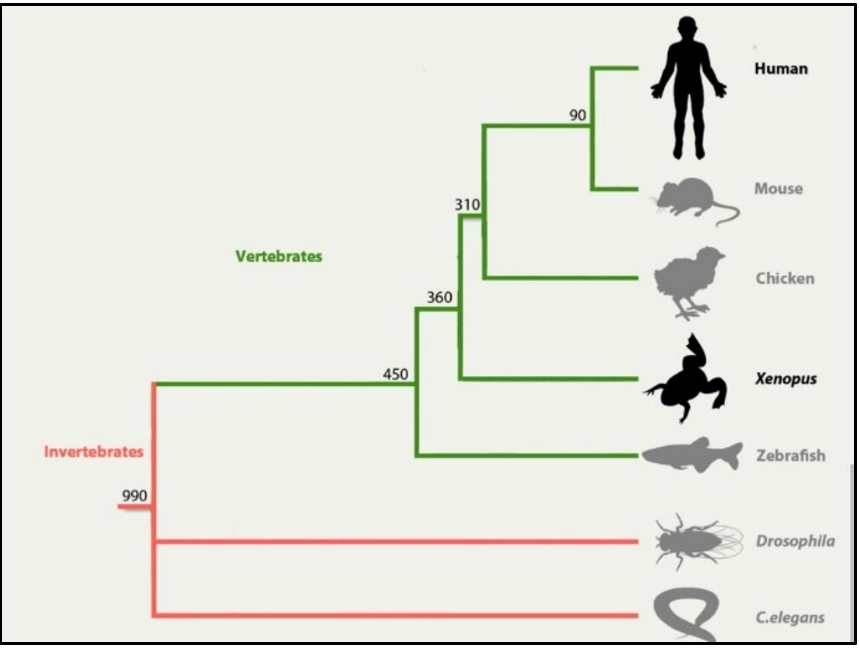
Modelling human diseases using the Xenopus species is an increasingly popular method to study vertebrate embryology and development, basic cell and molecular biology, genomics, neurobiology, and toxicology. This allows for the elucidation of the regulation mechanisms and interactive networks that affect the direct development of embryos, the adaptation process, and disease and malformation-causing dysregulations. Here we aim to analyze the possible kidney defects during the gene-knockout of CEP290 in X. tropicalis species. Our objectives are to produce sgRNA via de-novo synthesis from the constructed DNA template, to microinject synthesized sgRNA into embryos of Xenopus tropicalis, and to determin the success of the genome editing via T7-Endonuclease I assay and observation of genetically modified tadpoles for identifying any physical symptoms produced due to gene knockout. The data obtained from the embryos of X. tropicalis suggests that complete knock-out of the gene cep290 results in severe mutation that causes death.
References
Mitrikeski, P. T. (2012), Gene knockout: The technology of gene targeting, Third Annual Conference for Biotechnology and Transplantation, (September 2012), http://www.knockoutmouse.org/.
Fortriede et al. (2020b) Xenbase, Nucleic Acids Research, https://www.xenbase.org/other/static-xenbase/citingMOD.jsp (Accessed: 11 March 2022).
2020, T. M. I. (no date) CEP290 HGNC:29021, https://monarchinitiative.org/gene/HGNC:29021 (Accessed: 11 March 2022).
Nakayama, Takuya; Blitz, Ira L., Fish, Margaret B, Odeleye, Akinleye O, Manohar, Sumanth, Cho, Ken W.Y., Grainger, R. M. (2014), Cas 9 based genome editing in xenopus tropicalis.pdf, Methods Enzymol, 546, pp. 355–375. DOI: https://doi.org/10.1016/B978-0-12-801185-0.00017-9
Ford, K., McDonald, D. and Mali, P. (2019), Functional Genomics via CRISPR–Cas, Journal of Molecular Biology, pp. 48–65. doi: 10.1016/j.jmb.2018.06.034. DOI: https://doi.org/10.1016/j.jmb.2018.06.034
Fortriede et al. (2020a) About Xenopus, Nucleic Acids Research, https://www.xenbase.org/anatomy/intro.do (Accessed: 22 February 2022).
Joseph Sambrook, D. W. R. (1989), Molecular Cloning_ A Laboratory Manual (PDFDrive).pdf.
Blackburn, A. T. M. and Miller, R. K. (2019), Modeling congenital kidney diseases in Xenopus laevis, DMM Disease Models and Mechanisms. doi: 10.1242/dmm.038604 DOI: https://doi.org/10.1242/dmm.038604
Sharp, J. A. et al. (2011), Functional analysis of the microtubule-interacting transcriptome, Molecular Biology of the Cell, 22(22), pp. 4312–4323. doi: 10.1091/mbc.E11-07-0629. DOI: https://doi.org/10.1091/mbc.e11-07-0629
Ringers, C., Olstad, E. W. and Jurisch-Yaksi, N. (2020), The role of motile cilia in the development and physiology of the nervous system, Philosophical Transactions of the Royal Society B: Biological Sciences. doi: 10.1098/rstb.2019.0156. DOI: https://doi.org/10.1098/rstb.2019.0156
Wessely, O. and Obara, T. (2008), Fish and frogs: Models for vertebrate cilia signaling, Frontiers in Bioscience, pp. 1866–1880. doi: 10.2741/2806. DOI: https://doi.org/10.2741/2806
Bolton, P. M. and Beuchat, C. A. (1991), Cilia in the Urinary Bladder of Reptiles and Amphibians: A Correlate of Urate Production?, Copeia, 1991(3), p. 711. doi: 10.2307/1446397. DOI: https://doi.org/10.2307/1446397
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Nourin Shamnad

This work is licensed under a Creative Commons Attribution 4.0 International License.